Abstract
Purpose: The role of sunitinib and sorafenib in the treatment of metastatic renal cell carcinoma (mRCC) is reviewed.
Summary: Sunitinib malate is a potent inhibitor of vascular endothelial growth factor (VEGF) receptors, FMS-like tyrosine kinase 3 (FLT3), c-KIT, and platelet-derived growth factor (PDGF), which give the drug its direct antitumor and antiangiogenic properties. Sunitinib is currently approved as a second-line treatment of mRCC in patients who have either not responded to or who are not eligible to receive interleukin-2. Clinical trials of sunitinib have found similar rates of partial response, disease stabilization, and progression-free survival. Sorafenib inhibits VEGF receptors, PDGF receptors, FLT3, RAF-1, and BRAF in vitro and has been shown to prevent the growth of tumors but not to reduce tumor size. Sorafenib has been proven to improve survival in a novel randomized discontinuation trial and a Phase III randomized, placebo-controlled trial. No studies have directly compared the effectiveness of sunitinib to sorafenib in the treatment of advanced renal cell carcinoma. Sunitinib and sorafenib share a similar mechanism of action and primarily target tumor angiogenesis by inhibiting a variety of tyrosine kinases; the agents have similar toxicity, with the exception of an increased risk of hypertension associated with the use of sorafenib. Sorafenib does not result in tumor shrinkage, but sunitinib significantly reduces tumor size.
Conclusion: The tyrosine kinase inhibitors sorafenib and sunitinib offer improved outcomes for patients with mRCC, but they are far short of a cure. Despite the introduction of sorafenib and sunitinib, palliative care is still an acceptable treatment option for mRCC because of the disease's extremely poor prognosis.
Introduction
In 2007, 51,190 patients in the United States were expected to be diagnosed with kidney cancer, accounting for 2% of all cancers.[1] Renal cell carcinoma (RCC) comprises 90% of all kidney cancers. Patients at a high risk for developing RCC include heavy smokers, urban dwellers, and patients with genetic predispositions, such as Von Hippel-Lindau (VHL) disease, type 2 papillary RCC, and Birt-Hogg-Dube syndrome.[2] Other possible risk factors include polycystic kidney disease, diabetes mellitus, and chronic dialysis. The median age at RCC diagnosis is 65 years.[2] In two thirds of patients diagnosed with nonmetastatic RCC, the tumor is found incidentally. Only 10% of patients complain of RCC's main symptoms—flank pain, flank mass, and hematuria—until advanced stages of the disease.[2] Since symptoms are uncommon with early disease, many patients who seek medical advice have already developed metastatic RCC (mRCC). Common sites of metastases in these patients include the lungs, liver, bones, and brain.
Prognosis is based on stage as determined by the Tumor Node Metastasis staging classification. Patients without metastasis—stage I, II, or III RCC—have a predicted five-year survival rate of 91%, 74%, or 67%, respectively.[3] Even if the tumor has extended into the renal vein, the five-year survival rate is still 25-50%.[2] Early-stage RCC is potentially curable with nephrectomy.
Despite the effectiveness of a nephrectomy in treating RCC, 20-30% of patients' RCC will progress to mRCC.[4] Progression to metastatic disease within one year after nephrectomy confers the same survival rate as stage IV disease without nephrectomy.[2] In patients with stage IV disease, the median survival time is 10 months, and the one-, two-, and three-year survival rate is 42%, 20%, and 11%, respectively.[5] If metastatic disease develops more than two years after nephrectomy, then the patient has a more favorable prognosis with a five-year survival rate of 20%.[2] Nephrectomy is generally discouraged in patients with mRCC, as less than 1% of patients who undergo nephrectomy experience spontaneous remission and any benefit is far exceeded by the risk of surgery.[2]
RCC histologies are characterized by the cell of origin, morphology, and growth pattern. Five RCC subtypes have been identified: clear-cell, chromophilic, chromophobic, oncocytic, and collecting-duct tumors. The most common RCC histology is clear cell, accounting for 85% of all RCCs.[6] Clear-cell RCC is associated with a mutation in the gene that encodes for the VHL protein. VHL is a tumor-suppressor protein whose gene product acts like an antiangiogenic agent, inhibiting the actions of various hypoxia-induced genes. Individuals with VHL disease have a somatic or inherited mutation and do not produce the VHL protein. Inherited VHL disease occurs in every 1 of 36,000 births, and these individuals have a 30% chance of developing RCC, along with other systemic problems.[7] More commonly, a somatic mutation occurs, causing the VHL protein to be ineffective. Left unchecked, the hypoxia-inducible factors 1α and 2α promote the release of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), even in the presence of normoxia (Figure 1).[8] This allows angiogenesis and new vessel growth to proceed in the absence of hypoxia and injury.
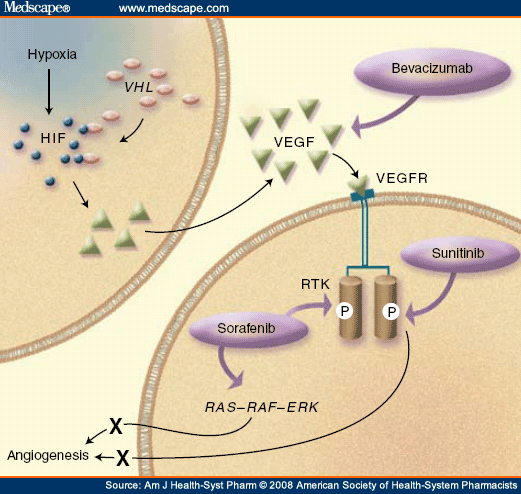
Due to the inactivation of VHL, overexpression of VEGF and PDGF, and increase in angiogenesis, clear-cell RCC is characterized by highly vascular primary tumors and metastases. Currently available treatment options, including chemotherapy, interleukin-2 (IL-2), interferon-alfa (IFN-α), and a combination of IL-2 and IFN-α, typically result in response rates of 5%, 20%, 12%, and 19%, respectively ( Table 1 ).[18-21] IL-2 is currently approved for the treatment of advanced RCC. Fourteen percent of patients achieve a complete response, the median duration of which has not been reached.[19] IL-2 is a first-line treatment for advanced RCC;[22] however, IL-2 cannot be given to many patients with advanced RCC because of significant toxicities that require drug administration and monitoring in an intensive care setting.[19] The National Comprehensive Cancer Network (NCCN) recommends palliative care as a first-line treatment option for patients with mRCC because of the limited and ineffective treatment options available for mRCC.[22]
Recently, angiogenesis inhibitors have been studied in the treatment of clear-cell mRCC. Bevacizumab, a monoclonal antibody that specifically targets soluble VEGF, has demonstrated improved progression-free survival time versus placebo but failed to show a benefit in overall survival in a Phase II randomized trial.[23] Bevacizumab is currently a second-line treatment for clear-cell mRCC.[22] Because of bevacizumab's promising results, other agents that target both VEGF and PDGF have been studied for the treatment of advanced RCC.
Unlike bevacizumab, which targets extracellular VEGF, tyrosine kinase inhibitors target the intracellular signaling pathways of VEGF receptors. Figure 1 shows how VEGF increases angiogenesis and the sites of action of various antiangiogenic agents. Tyrosine kinase inhibitors target VEGF receptors and a variety of receptors that rely on a tyrosine kinase component to function properly, including PDGF, FMS-like tyrosine kinase 3 (FLT3), RAF, and c-KIT.[24] The receptor structure consists of an extracellular binding site, a transmembrane region, and an intracellular tyrosine kinase domain. Binding-site activation induces stabilized receptor dimerization, which leads to increased tyrosine kinase activity. This catalyzes a series of reactions and signaling pathways that generally lead to proliferation.[10] The small-molecule tyrosine kinase inhibitors sorafenib and sunitinib inhibit the receptor activation.




